Projects at SEIS Consult
Current Projects:
- Eutectic Freeze Crystallisation
- Hydrothermal Technology
- Tank Cleaning market analyses and recommendations
- Chemical decontamination market analyses and recommendations
Recent Projects:
- Feasibility study Integrated Gas Services Australia / New Zealand / Papua New Guinea
- Developed Tank Cleaning Matrix to facilitate the selection of the most suitable non man-entry tank cleaning technology for crude and or heavy feedstock storage tanks.
Eutectic Freeze Crystallisation
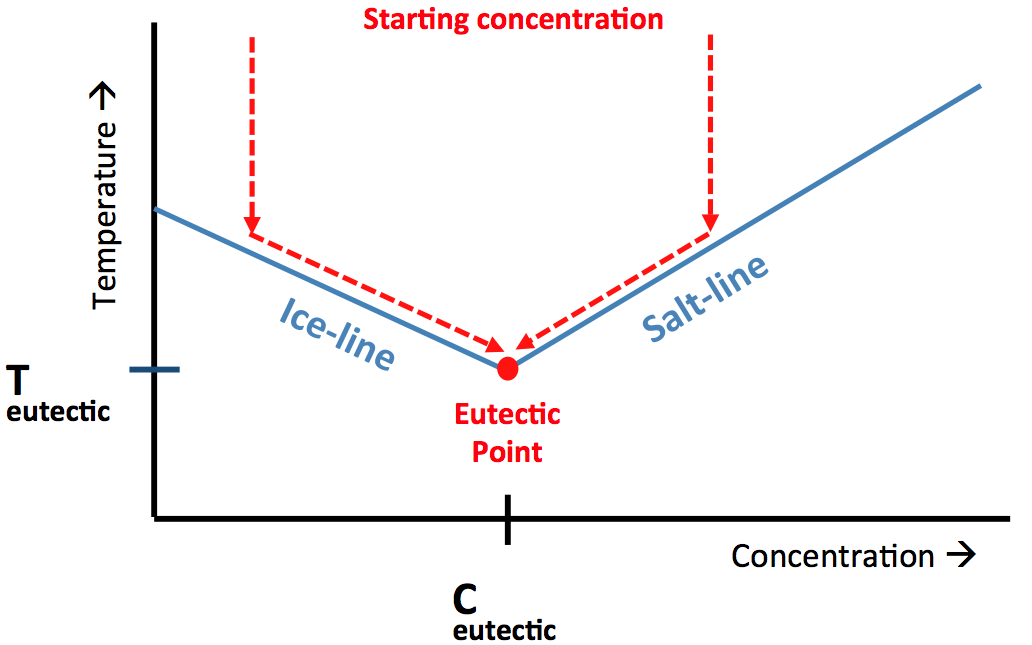
| Eutectic Freeze Crystallization – The Basics The basis of the eutectic freeze crystallization (EFC) process is the existence of the eutectic point. The eutectic point is a characteristic point in the phase diagram of a salt-water mixture. At the eutectic point an equilibrium exists between ice, salt and a solution with a specific concentration. This specific concentration is called the eutectic concentration and the temperature at which this equilibrium is found is the eutectic temperature.
Figure 1. A typical phase diagram for a salt-water system showing the eutectic point. See description in text below. Figure 1 shows a typical phase diagram for a binary system (salt-water). In the case that an aqueous solution has exactly the eutectic concentration cooling the solution down towards its eutectic temperature will lead to the simultaneous crystallization of both ice and salt. However, in practice it is common that a solution has a salt concentration that is lower or higher than the eutectic concentration. In the former case ice will crystallize first when the temperature is decreased. Due to the formation of ice the salt concentration in the remaining liquid (the mother liquor) increases, which leads to a decrease in freezing point and by continued cooling the ice line is followed till the eutectic point is reached. This is represented by path A in figure 1. When the original salt concentration is higher than the eutectic concentration the opposite happens; first salt is crystallizing till the salt concentration of the mother liquor decreases to the eutectic concentration, from that moment on also ice will be formed. The locations of the eutectic points in a water-salt mixture is dependent on the type of ions in solution and can vary over a broad range (both in temperature as well as concentrations) for different systems. Eutectic Freeze Crystallization – The Process
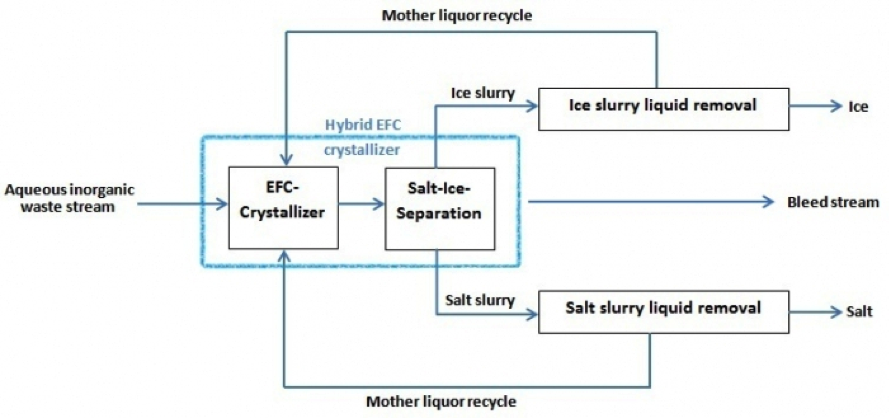
Figure 2. Schematic block scheme of the EFC process. A bleed stream is not necessarily required but can be added for process optimization. Fields of Application
The benefits of using the EFC process are in many cases twofold. In the first place the problem of having a wastewater stream (e.g. disposal costs, loss of water, etc.) is solved. Secondly, the user of the EFC process is able to recover (valuable) products from its wastewater. The latter characteristic makes that the EFC process is not only a wastewater treatment technology, it can be seen as a high-quality salt production process as well.
SEIS Consult is engaged by EFC Separations BV as the first point of contact in the Asia-Pacific region. |
SEIS Consult Pty Ltd Pine Mountain, QLD 4306, Australia Phone +61 (0) 400 363 829 Email: info@seisconsult.com © 2017 SEIS Consult ABN: 67 632 447 262 ACN: 632 447 262 Terms of use / Privacy Policy / Site Map |
Find us on LinkedIn
|